饲料能量密度和投喂水平对吉富罗非鱼生长和健康的影响
摘要: 为探讨饲料能量密度(DED)和投喂水平(DFR)对鱼类生长和健康的影响, 本试验采用2×2双因子设计, 设置2个DED(对照组和高糖高脂组)和2个DFR(1倍和1.2倍表观饱食投喂对照组饲料的能量水平), 研究DED和DFR对吉富罗非鱼[Oreochromis niloticus, (14.59±0.06) g]生长性能、饲料利用、体成分、血液学指标和抵抗无乳链球菌(Streptococcus agalactiae)感染能力的影响。试验以40d为1个周期, 持续2个周期(周期Ⅰ和周期Ⅱ)。研究结果发现, DED和DFR均未影响试验鱼的饲料效率(P>0.05)。DED未影响试验鱼的生长(P>0.05)。高DFR提升了试验鱼的末重(P<0.05), 但降低了周期Ⅱ的蛋白质沉积率(P<0.05)。DED和DFR均未影响试验鱼的肥满度(P>0.05)。在周期Ⅰ, 高DED仅增加了试验鱼的脏体比(P<0.05); 但在周期Ⅱ, DED和DFR对试验鱼的肠体比和脏体比也产生了显著影响(P<0.05)。在周期Ⅰ, 高DED引起了去内脏全鱼、内脏团粗脂肪含量显著升高(P<0.05), 高DFR引起了肝脏粗脂肪含量显著升高(P<0.05); 而在周期Ⅱ, 高DED和DFR均引起了去内脏全鱼、肝脏和肌肉粗脂肪含量显著升高(P<0.05)。在试验期内, 高DED和DFR显著升高了试验鱼的血清甘油三酯、丙二醛含量(P<0.05)和周期Ⅰ的血细胞比容(P<0.05), 降低了周期Ⅱ的白细胞计数(P<0.05); 高DED升高了试验鱼的血清碱性磷酸酶活性和周期Ⅱ的血清胆固醇水平(P<0.05), 降低了周期Ⅰ的过氧化氢酶和谷胱甘肽过氧化物酶活性(P<0.05); 高DFR显著升高周期Ⅰ试验鱼的血糖水平(P<0.05)。无乳链球菌感染试验发现, 在周期Ⅰ, DED和DFR对试验鱼的成活率无显著影响(P>0.05); 可在周期Ⅱ, 高DFR造成试验鱼成活率显著降低(P<0.05)。以上结果表明, 高DFR能提高罗非鱼的生长速度, 但是会增加鱼体的脂肪沉积, 降低鱼体健康水平; 高DED更易于鱼体脂肪沉积, 不利于鱼体健康。
关键词: 饲料能量密度 / 投喂量 / 罗非鱼 / 生长 / 体成分 / 血液学 / 抗病力Abstract: To investigate the effects of dietary energy density (DED) and dietary feed ration (DFR) on fish growth and health, a 2×2 factorial experiment was designed, including 2 DED (control diet and high carbohydrate and fat diet) and 2 DFR (1 and 1.2 times energy level of fish fed to control diet apparent satiation). To evaluate the effects of DED and DFR on growth performance, feed utilization, body composition, hematological indices and resistant to Streptococcus agalactiae infection of genetically improved farmed tilapia, Oreochromis niloticus (14.59±0.06) g, the study set 40d as a cycle for 2 cycles (period Ⅰ and Ⅱ). The results showed that DED and DFR did not affect the feed efficiency (P>0.05), and DED did not affect the fish growth performance (P>0.05). High DFR improved the final mean weight (P<0.05), but reduced protein retention during period Ⅱ (P<0.05). DED and DFR had no impact on the conditional factor (P>0.05); in period Ⅰ, high DED only induced the viscerasomatic index (P<0.05), but in period Ⅱ, DED and DFR had significant effects on intestinal-somatic index and viscerasomatic index (P<0.05). High DED increased crude lipid content in the eviscerated whole fish and visceral mass (P<0.05); in period Ⅰ, high DFR enhanced crude lipid content in the liver (P<0.05), and in period Ⅱ, high DED and DFR induced crude lipid content in the eviscerated whole fish, liver, and muscle (P<0.05). High DED and DFR increased in the serum triglycerides and malondialdehyde content (P<0.05), and hematocrit in period Ⅰ(P<0.05), but it decreased the white cell count in period Ⅱ (P<0.05); high DED increased the serum alkaline phosphatase activity, and serum cholesterol (period Ⅱ) (P<0.05), but decreased the catalase and glutathione peroxidase activity (period Ⅰ) (P<0.05); high DFR increased the serum glucose levels (P<0.05). After infection with S. Agalactiae, DED and DFR did not affect the survival rate in period Ⅰ (P>0.05); however, high DFR decreased the survival rate in period Ⅱ (P<0.05). In conclusion, these results suggested that high DFR could improve the growth rate of tilapia, increase the fat deposition and reduce the health level of fish, and DED is more prone to fat deposition to impact fish health.
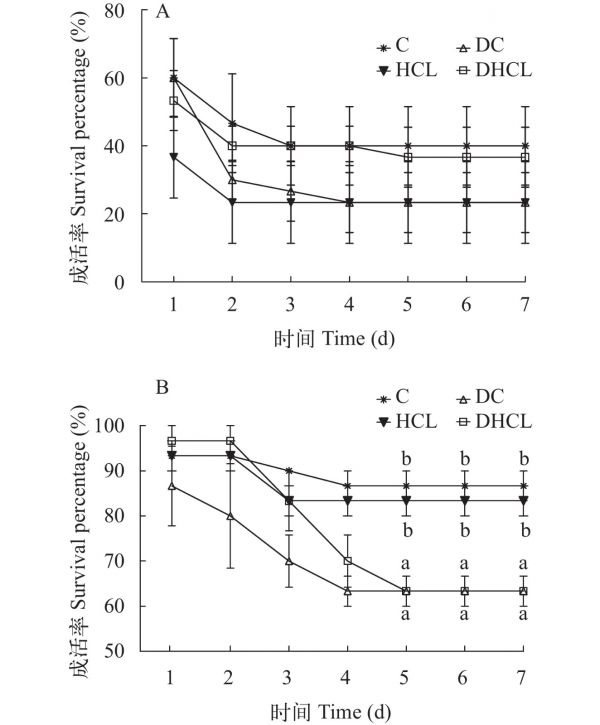
图 1 无乳链球菌攻毒中试验鱼的累计成活率
A. 周期Ⅰ结束后攻毒结果; B. 周期Ⅱ结束后攻毒结果; 同一时间点具不同小写字母表示差异显著(P<0.05)
Figure 1. Cumulative survival percentage of GIFT challenged by S. agalactiae
A. challenge results after period Ⅰ; B. challenge results after period Ⅱ. Different lowercase letters at the same time point indicate significant differences (P<0.05)
表 1 试验饲料配方和营养成分
Table 1 Formulation and composition of the experimental diets (%)
原料成分Ingredient饲料Diet对照Control高糖高脂High carbohydrate-high lipid 酪蛋白Casein31.0031.00明胶Gelatin 7.00 7.00糊精Dextrin32.0042.00微晶纤维素Microcrystalline cellulose16.65 0.65大豆油Soybean oil 3.00 6.00玉米油Corn oil 3.00 6.00维生素预混料Vitamin premix* 1.00 1.00矿物质预混料Mineral premix* 1.00 1.00磷酸二氢钙Monocalcium phosphate 2.00 2.00氯化胆碱Choline chloride 0.25 0.25牛磺酸Taurine 1.00 1.00沸石粉Zeolite powder 1.90 1.90二氧化钛Titanium dioxide 0.20 0.20成分分析Proximate composition (dry matter, %)粗蛋白Crude protein34.2934.69粗脂肪Crude lipid 6.2612.63灰分Ash 4.61 4.64总能Total energy (kJ/g)21.9423.92可消化能Digestible energy (kJ/g)17.2620.76 注: *维生素预混料由下列成分组成(g/kg预混料): 硫胺素盐酸盐. 5; 核黄素. 5; 泛酸钙. 10; D-生物素. 0.003; 盐酸吡哆醇. 4; 叶酸. 1.5; 肌醇. 200; L-维生素C-2-磷酸镁. 3.95; 烟酸. 6.05; α-维生素E醋酸酯. 50; 维生素K3. 4; 视黄醇乙酸酯. 0.4; 维生素D3. 160000 IU; 添加微晶纤维素至1 kg; *矿物质预混料由下列成分组成(g/kg 预混料): 磷酸二氢钙. 135; 乳酸钙. 327; 硫酸亚铁. 2.125; 硫酸镁. 137; 磷酸二氢钠. 87.2; 氯化钠. 43.5; 氯化铝. 0.15; 碘酸钾. 0.125; 氯化钾. 75; 氯化铜. 0.1; 硫酸锰. 0.8; 氯化钴. l; 硫酸锌. 3; 添加微晶纤维素至1 kgNote: * Vitamin premix consists of the following ingredients (g/kg premix): thiamine hydrochloride. 5; riboflavin. 5; calcium pantothenate. 10; d-biotin. 0.003; pyridoxine hydrochloride. 4; folic acid. 1.5; inositol. 200; magnesium L-vitamin C-2-phosphate. 3.95; niacin. 6.05; vitamin E acetate. 50; vitamin K3. 4; retinol acetate. 0.4; vitamin D3. 160000 IU; add microcrystalline cellulose to 1 kg; *Mineral premix consists of the following ingredients (g/kg premix): calcium dihydrogen phosphate. 135; calcium lactate. 327; ferrous sulfate. 2.125; magnesium sulfate. 137; sodium dihydrogen phosphate. 87.2; sodium chloride. 43.5; aluminium chloride. 0.15; potassium iodate. 0.125; potassium chloride. 75; copper chloride. 0.1; manganese sulfate. 0.8; cobalt chloride. 1; zinc sulfate. 3; add microcrystalline cellulose to 1 kg
表 2 饲料能量密度和投喂水平对试验鱼生长和饲料利用效率的影响
Table 2 Effects of dietary energy density and feed ration on grow performance and feed utilization of GIFT (n=3)
指标Index组别Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy
density投喂量
Feed
ration交互作用
Interaction 周期Ⅰ
Period Ⅰ初始平均体重Wi (g)14.77±0.0514.51±0.1414.66±0.0914.43±0.07末平均体重Wf (g)40.84±1.20a46.39±0.99b40.64±1.94a47.82±1.19b0.5970.0000.490成活率SR (%)98.99±1.1196.67±3.3396.11±0.5695.56±1.110.3270.4770.667特定生长率SGR (%/d)3.39±0.10a3.87±0.10b3.40±0.11a3.99±0.08b0.5330.0010.595增重率WGR (%)176.56±8.22a219.98±9.38b177.43±9.36a231.49±8.05b0.5000.0010.561饲料效率FE1.02±0.031.01±0.051.02±0.061.08±0.050.5290.5940.436蛋白质沉积率PRE (%)49.49±1.1649.27±3.0449.80±3.2151.53±1.420.6050.7600.695周期Ⅱ
Period Ⅱ初始平均体重Wi (g)62.41±0.73a73.25±0.51b62.57±0.62a73.29±1.43b0.9160.0000.949末平均体重Wf (g)121.10±1.82a141.68±2.19b121.12±3.37a135.21±2.12b0.2240.0000.221成活率SR (%)100100100100特定生长率SGR (%)1.66±0.021.65±0.041.65±0.081.53±0.090.9710.4790.893增重率WGR (%)84.95±5.3690.88±6.1693.39±6.77104.46±6.490.3720.3640.427饲料效率FE0.80±0.020.78±0.030.81±0.050.77±0.040.3970.3720.433蛋白质沉积率PRE (%)35.97±3.04b27.78±0.38a34.79±1.03ab31.87±1.74ab0.5230.0340.261 注: 表格中同行平均数上标不同表示差异显著(P<0.05); 下同Note: The mean values in the same row with different letters are significantly different (P<0.05). The same applies below
表 3 饲料能量密度和投喂水平对试验鱼形体指标的影响
Table 3 Effects of dietary energy density and feed ration on physical parameters of GIFT (n=3)
指标Index组别Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy density投喂水平
Feed ration交互作用
Interaction 周期Ⅰ
Period Ⅰ肠体比ISI7.41±0.318.40±0.477.54±0.297.69±0.400.4610.1650.299肥满度CF (g/cm3)3.43±0.013.51±0.053.58±0.083.58±0.080.1050.5200.530脏体比VSI 9.49±0.40a9.66±0.16a10.14±0.19ab10.89±0.08b0.0040.0920.258肝体比HSI 1.94±0.211.84±0.031.84±0.251.86±0.030.8150.8010.711周期Ⅱ
Period Ⅱ肠体比ISI7.89±0.26a9.60±0.21b7.91±0.43a8.20±0.27ab0.0380.0290.100肥满度CF (g/cm3)4.34±0.114.30±0.254.44±0.134.38±0.130.9370.7520.964脏体比VSI10.33±0.19a13.68±0.14c12.05±0.35b15.08±0.26d0.0000.0000.543肝体比HSI1.80±0.18a3.69±0.09b2.46±0.30a3.95±0.37b0.1120.0000.471
表 4 饲料能量密度和投喂水平对周期Ⅰ试验鱼体成分的影响 (n=3, %湿重)
Table 4 Effects of dietary energy density and feed ration on body composition of GIFT in period Ⅰ (n=3, % wet weight)
指标Index组别Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy density投喂量
Feed ration交互作用
Interaction 去内脏全鱼
The eviscerated whole fish水分Moisture72.36±0.19b72.12±0.50b70.06±0.28a70.33±0.22a0.0000.9630.438粗脂肪Crude lipid6.62±0.35a6.44±0.54a8.43±0.32b8.66±0.10b0.0010.9560.583粗蛋白Crude protein16.24±0.1416.40±0.1516.56±0.2516.35±0.380.5960.9320.476灰分Ash4.35±0.11ab4.53±0.17b4.14±0.11ab3.98±0.07a0.0140.9070.199肝脏Liver水分Moisture68.70±0.7068.61±0.5366.79±0.5865.82±0.780.0090.4590.531粗脂肪Crude lipid9.98±0.9113.74±0.0711.94±0.3713.47±1.400.3510.0150.229粗蛋白Crude protein12.93±0.4712.76±0.7011.78±0.8312.13±0.360.1910.8860.684内脏团
Visceral
mass*水分Moisture74.26±1.8874.17±2.7168.07±2.5466.05±2.410.0180.6730.700粗脂肪Crude lipid8.87±0.06a9.08±0.50a17.82±1.57b19.84±1.88b0.0000.3980.493粗蛋白Crude protein8.36±0.178.28±0.358.08±0.218.56±0.300.9990.4710.320肌肉Muscle水分Moisture77.66±0.3177.09±0.4577.05±0.3276.69±0.590.2770.3220.814粗脂肪Crude lipid0.94±0.101.06±0.311.13±0.111.19±0.180.5010.5970.818粗蛋白Crude protein17.48±0.2017.72±0.2118.20±0.6918.44±0.510.1540.6200.999 注: *内脏团不包含肝脏; 下同Note: *Visceral masses do not contain the liver. The same applied below
表 5 饲料能量密度和投喂水平对周期Ⅱ试验鱼体成分的影响 (n=3, %湿重)
Table 5 Effects of dietary energy density and feed ration on body composition of GIFT in period Ⅱ (n=3, % wet weight)
指标Index组别Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy density投喂量
Feed ration交互作用
Interaction 去内脏全鱼
The
eviscerated
whole fish水分Moisture69.04±0.20c67.69±0.55b67.96±0.09b66.13±0.19a0.0030.0010.469粗脂肪Crude lipid 8.64±0.16a9.99±0.18a11.49±0.24b12.00±0.40b0.0000.0080.148粗蛋白Crude protein17.55±0.3516.69±0.5816.77±0.2116.72±0.460.4610.3140.366灰分Ash 4.57±0.094.31±0.014.63±0.094.58±0.210.3170.2440.411肝脏Liver水分Moisture69.36±2.9267.47±0.7064.78±0.0265.02±1.260.2280.6260.531粗脂肪Crude lipid13.97±0.27a15.11±0.26a14.93±0.57a18.51±0.13b0.0000.0000.008粗蛋白Crude protein14.84±0.6813.94±0.8913.26±0.7811.78±0.700.1070.1590.717内脏团
Visceral
mass*水分Moisture65.75±4.43a67.54±2.54a47.04±3.12b43.36±2.87b0.0010.7830.434粗脂肪Crude lipid19.05±0.64a25.11±1.55b20.36±0.17a27.64±1.24b0.1310.0000.606粗蛋白Crude protein 6.99±0.436.26±0.235.32±0.385.78±0.450.0710.7300.157肌肉Muscle水分Moisture77.38±0.3777.13±0.2776.87±0.2776.20±0.150.0770.1390.468粗脂肪Crude lipid 0.77±0.18a1.63±0.15b1.40±0.07b1.82±0.07b0.0130.0010.126粗蛋白Crude protein18.46±0.35a18.21±0.05a19.73±0.37b20.66±0.40b0.0020.3270.106
表 6 饲料能量密度和投喂水平对试验鱼血液生理指标的影响
Table 6 Effects of dietary energy density and feed ration on blood physiological indices of GIFT (n=3)
指标Index组别Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy density投喂量
Feed ration交互作用
Interaction 周期Ⅰ
Period Ⅰ血红蛋白HB (g/L)187.33±18.67236.67±5.46231.33±8.11230.67±3.710.1140.2090.558红细胞数RBC (×1012)14.00±3.9415.112±1.6719.05±2.8214.82±1.560.4010.1290.627白细胞数WBC (×1010)23.50±2.0214.33±1.4522.50±3.3319.33±12.110.7630.4260.189血细胞比容HCT (%)37.56±0.4939.89±0.9846.02±2.3347.22±3.500.0070.0120.058周期Ⅱ
Period Ⅱ血红蛋白HB (g/L)179.67±13.72188.33±13.86182.67±5.46180.33±2.030.9270.7640.604红细胞数RBC (×1012)14.38±1.2311.27±3.5011.43±0.8017.35±1.850.2150.5260.065白细胞数WBC (×1010)51.00±4.36b42.67±5.83b49.00±0.76b16.50±1.76a0.0060.0010.012血细胞比容HCT (%)38.26±2.5132.16±0.3838.09±4.4431.12±1.300.3740.2170.361
表 7 饲料能量密度和投喂水平对周期Ⅰ试验鱼血液生化指标的影响
Table 7 Effects of dietary energy density and feed ration on blood biochemical indices of GIFT in period Ⅰ (n=3)
指标Index组别Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy density投喂量
Feed ration交互作用
Interaction 谷草转氨酶AST (U/L)170.33±5.55161.33±2.33171.00±10.79179.33±6.060.2120.9630.243谷丙转氨酶ALT (U/L)54.67±4.1054.00±4.3657.00±4.3658.67±3.920.4280.9080.788碱性磷酸酶ALP (U/L)30.33±1.4534.00±1.5342.00±3.5141.67±3.840.0090.5690.497乳酸脱氢酶LD (U/L)7.00±1.53a14.67±1.33b17.33±0.33b17.00±1.00b0.0010.0120.008甘油三酯TGK (mmol/L)4.03±0.24a5.44±0.35bc4.82±0.08b5.77±0.10c0.0360.0010.330胆固醇TCHO (mmol/L)4.62±0.245.17±0.344.92±0.325.55±0.030.2380.0560.889高密度脂蛋白胆固醇HDLC (mmol/L)1.33±0.091.40±0.091.43±0.181.55±0.050.3060.4030.850低密度脂蛋白胆固醇LDLC (mmol/L)0.58±0.061.16±0.370.91±0.091.15±0.070.4310.0680.414白蛋白ALB (g/L)10.33±0.3310.33±0.3310.00±0.5811.00±0.580.7330.2090.558总蛋白TP (g/L)40.33±1.2041.67±0.3338.00±3.2139.50±1.230.2910.1290.627尿素氮BUN (mmol/L)0.56±0.040.68±0.040.69±0.070.53±0.070.8870.4260.189血糖GLU (mmol/L)7.84±0.878.11±0.497.44±0.788.57±0.220.9680.0120.058
表 8 饲料能量密度和投喂水平对周期Ⅱ试验鱼血清生化指标的影响
Table 8 Effects of dietary energy density and feed ration on serum biochemical indices of GIFT in period Ⅱ (n=3)
指标Index组别Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy density投喂量
Feed ration交互作用
Interaction 谷草转氨酶AST (U/L)76.67±11.62102.67±4.6794.00±5.0393.33±2.400.1320.1020.088谷丙转氨酶ALT (U/L)59.33±6.9651.00±5.2042.67±5.2144.00±5.770.2390.5650.431碱性磷酸酶ALP (U/L)26.67±3.7127.33±2.4036.00±6.0043.33±1.330.0430.3220.404乳酸脱氢酶LD (U/L)212.00±14.47a452.00±26.86b428.00±41.00b455.33±26.39b0.0010.0020.006甘油三酯TGK (mmol/L)2.91±0.49a4.65±0.22b5.49±0.39b6.76±0.04c0.0000.0020.498胆固醇TCHO (mmol/L)5.03±0.20a5.50±0.23a8.12±1.01b7.73±0.62b0.0160.9500.500高密度脂蛋白胆固醇HDLC (mmol/L)2.71±0.192.39±0.172.32±0.172.58±0.170.4290.8550.136低密度脂蛋白胆固醇LDLC (mmol/L)2.60±0.182.86±0.243.37±0.253.67±0.280.0520.2760.926白蛋白ALB (g/L)12.67±0.67b10.00±0.00a11.33±0.67ab12.67±0.67b0.0320.2820.009总蛋白TP (g/L)54.67±2.4052.00±6.4356.67±3.7163.33±1.330.2880.6270.272尿素氮BUN (mmol/L)1.76±0.171.47±0.142.03±0.171.78±0.080.1250.0940.890血糖GLU (mmol/L)4.15±0.50a4.38±0.09a5.95±0.41ab6.91±0.63b0.0080.2250.445
表 9 饲料能量密度和投喂水平对试验鱼血清抗氧化指标的影响
Table 9 Effects of dietary energy density and feed ration on serum antioxidant indices of GIFT (n=3)
指标Index组别 Group双因素方差分析Two way ANOVACDCHCLDHCL能量密度Energy density投喂量
Feed ration交互作用
Interaction 周期Ⅰ
Period ⅠSOD (U/mL)47.93±0.9551.81±7.9244.91±4.7555.08±4.410.9810.0530.048CAT (U/mL)148.43±22.48b156.87±6.48b84.01±5.49a51.91±14.78a0.0000.3640.652GSH-Px (U/mL)6.95±0.87b3.27±0.65a3.45±0.68a2.76±0.43a0.0180.4400.802周期Ⅱ
Period ⅡSOD (U/mL)47.38±5.1441.43±1.3646.32±5.8643.76±4.510.7950.3770.720GSH-Px (U/mL)18.89±4.6011.91±0.8315.62±0.4926.32±4.330.0650.6080.026CAT (U/mL)22.76±1.4920.83±2.4726.12±3.5318.86±2.610.3150.1180.339TAOC (U/mL)6.49±0.843.51±0.543.99±0.734.80±0.680.0730.1630.028MDA (nmol/mL)9.37±1.24a18.19±1.28b14.94±1.00b18.66±1.59b0.0030.0010.084 注: SOD. 超氧化物歧化酶; CAT. 过氧化氢酶; GSH-Px. 谷胱甘肽过氧化物酶; TAOC. 总抗氧化能力; MDA. 丙二醛; 表格中同行平均数上标不同表示差异显著(P<0.05)Note: SOD. superoxide dismutase; CAT. catalase; GSH-Px. glutathione peroxidase; TAOC. Total antioxidant capacity; MDA. malondialdehyde. The mean values in the same row with different letters are significantly different (P<0.05) [1] 刘作华, 杨飞云, 孔路军, 等. 日粮能量水平对生长育肥猪肌内脂肪含量以及脂肪酸合成酶和激素敏感脂酶mRNA表达的影响 [J]. 畜牧兽医学报, 2007, 38(9): 934-941. doi: 10.3321/j.issn:0366-6964.2007.09.009
Liu Z H, Yang F Y, Kong L J, et al. Efects of dietary energy level on the content of intramuscular fat and mrna expression for fatty acid synthase and hormone-sensitive lipase in growing-finishing pigs [J]. Acta Veterinaria et Zootechnica Sinica, 2007, 38(9): 934-941. doi: 10.3321/j.issn:0366-6964.2007.09.009
[2]Fuentes E, Fuentes F, Vilahur G, et al. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome [J]. Mediators of Inflammation, 2013(2): 136584.
[3] 徐成斌. 代谢综合征 [J]. 国际内分泌代谢杂志, 2005, 25(1): 3-6. doi: 10.3760/cma.j.issn.1673-4157.2005.01.002Xu C B. Metabolic syndrome [J]. Section of Endocrinology Foreign Medical Sciences, 2005, 25(1): 3-6. doi: 10.3760/cma.j.issn.1673-4157.2005.01.002
[4]Stolarczyk E. Adipose tissue inflammation in obesity: a metabolic or immune response [J]? Current Opinion in Pharmacology, 2017(37): 35-40.
[5]Nieman D C, Henson D A, Nehlsen-Cannarella S L, et al. Influence of obesity on immune function [J]. Journal of the American Dietetic Association, 1999, 99(3): 294-299. doi: 10.1016/S0002-8223(99)00077-2
[6]Giovanni D P, Franco S. Obesity as a major risk factor for cancer [J]. Journal of Obesity, 2013(13): 291546.
[7] 杜震宇. 养殖鱼类脂肪肝成因及相关思考 [J]. 水产学报, 2014, 38(9): 1628-1638.Du Z Y. Causes of fatty liver in farmed fish: a review and new perspectives [J]. Journal of Fisheries of China, 2014, 38(9): 1628-1638.
[8]National Research Council. Nutrient Requirements of Fish and Shrimp [M]. Washington, DC: The National Academy Press, 2011: 36, 38.
[9]Xie D, Yang L, Yu R, et al. Effects of dietary carbohydrate and lipid levels on growth and hepatic lipid deposition of juvenile tilapia, Oreochromis niloticus [J]. Aquaculture, 2017, 479(1): 696-703.
[10]Alcorn S W, Pascho R J, Murray A L, et al. Effects of ration level on immune functions in chinook salmon (Oncorhynchus tshawytscha) [J]. Aquaculture, 2003, 217(1): 529-545.
[11]Li X F, Xu C, Tian H Y, et al. Feeding rates affect stress and non-specific immune responses of juvenile blunt snout bream Megalobrama amblycephala subjected to hypoxia [J]. Fish & Shellfish Immunology, 2016, 49(Supplement C): 298-305.
[12]Lin Y H, Shiau S Y. Dietary lipid requirement of grouper, Epinephelus malabaricus, and effects on immune responses [J]. Aquaculture, 2003, 225(1): 243-250.
[13]Torfi Mozanzadeh M, Yavari V, Marammazi J G, et al. Optimal dietary carbohydrate-to-lipid ratios for silvery-black porgy (Sparidentex hasta) juveniles [J]. Aquaculture Nutrition, 2017, 23(3): 470-483. doi: 10.1111/anu.12415
[14]Feng L, Ni P J, Jiang W D, et al. Decreased enteritis resistance ability by dietary low or excess levels of lipids through impairing the intestinal physical and immune barriers function of young grass carp (Ctenopharyngodon idella) [J]. Fish & Shellfish Immunology, 2017, 67(Supplement C): 493-512.
[15]Wu C, Ye J, Gao J E, et al. The effects of dietary carbohydrate on the growth, antioxidant capacities, innate immune responses and pathogen resistance of juvenile Black carp Mylopharyngodon piceus [J]. Fish & Shellfish Immunology, 2016, 49(Supplement C): 132-142.
[16]Wang M, Lu M. Tilapia polyculture: a global review [J]. Aquaculture Research, 2016, 47(8): 2363-2374. doi: 10.1111/are.12708
[17]Yuan Y, Yuan Y, Dai Y, et al. Technical efficiency of different farm sizes for tilapia farming in China [J]. Aquaculture Research, 2020, 51(1): 307-315. doi: 10.1111/are.14376
[18]Ng W K, Romano N. A review of the nutrition and feeding management of farmed tilapia throughout the culture cycle [J]. Reviews in Aquaculture, 2013, 5(4): 220-254. doi: 10.1111/raq.12014
[19]Richter H, Lückstädt C, Focken U, et al. Evacuation of pelleted feed and the suitability of titanium (Ⅳ) oxide as a feed marker for gut kinetics in Nile tilapia [J]. Journal of Fish Biology, 2003, 63(5): 1080-1099. doi: 10.1046/j.1095-8649.2003.00225.x
[20]Zhu C, Yu L, Liu W, et al. Dietary supplementation with Bacillus subtilis LT3-1 enhance the growth, immunity and disease resistance against Streptococcus agalactiae infection in genetically improved farmed tilapia, Oreochromis niloticus [J]. Aquaculture Nutrition, 2019, 25(6): 1241-1249. doi: 10.1111/anu.12938
[21]Schrama J W, Saravanan S, Geurden I, et al. Dietary nutrient composition affects digestible energy utilisation for growth: a study on Nile tilapia (Oreochromis niloticus) and a literature comparison across fish species [J]. The British Journal of Nutrition, 2012, 108(2): 277-289. doi: 10.1017/S0007114511005654
[22]Xie S, Cui Y, Yang Y, et al. Effect of body size on growth and energy budget of Nile tilapia, Oreochromis niloticus [J]. Aquaculture, 1997, 157(1): 25-34.
[23]Yang S, Zhai S W, Shepherd B S, et al. Determination of optimal feeding rates for juvenile lake sturgeon (Acipenser fulvescens) fed a formulated dry diet [J]. Aquaculture Nutrition, 2019, 25(6): 1171-1182. doi: 10.1111/anu.12932
[24] 李红燕, 巫丽云, 董博, 等. 饲料糖和脂水平对团头鲂生长性能及血浆代谢物的影响 [J]. 水生生物学报, 2021, 45(4): 756-763.Li H Y, Wu L Y, Dong B, et al. Effects of dietary carbohydrate and lipid levels on growth performance and plasma metabolites in juvenile blunt snout bream [J]. Acta Hydrobiologica Sinica, 2021, 45(4): 756-763.
[25]Hillestad, Johnsen, Austreng, et al. Long-term effects of dietary fat level and feeding rate on growth, feed utilization and carcass quality of Atlantic salmon [J]. Aquaculture Nutrition, 1998, 4(2): 89-97. doi: 10.1046/j.1365-2095.1998.00051.x
[26]Liu W, Wen H, Luo Z. Effect of dietary protein levels and feeding rates on the growth and health status of juvenile genetically improved farmed tilapia (Oreochromis niloticus) [J]. Aquaculture International, 2018, 26(1): 153-167. doi: 10.1007/s10499-017-0202-6
[27]Magnuson A M, Regan D P, Booth A D, et al. High-fat diet induced central adiposity (visceral fat) is associated with increased fibrosis and decreased immune cellularity of the mesenteric lymph node in mice [J]. European Journal of Nutrition, 2020, 59(4): 1641-1654. doi: 10.1007/s00394-019-02019-z
[28] 张彩霞, 陈文, 黄艳群, 等. 限饲对哈巴德肉鸡肠道结构的影响 [J]. 江西农业大学学报(自然科学版), 2010, 32(4): 677-682.Zhang C X, Chen W, Huang Y Q, et al. Effect of feed restriction on harbord broilers’ intestinal structure [J]. Acta Agriculturae Universitatis Jiangxiensis (Natural Sciences Edition), 2010, 32(4): 677-682.
[29]Fazio F. Fish hematology analysis as an important tool of aquaculture: A review [J]. Aquaculture, 2019(500): 237-242.
[30]Kawamoto R, Tabara Y, Kohara K, et al. Hematological parameters are associated with metabolic syndrome in Japanese community-dwelling persons [J]. Endocrine, 2013, 43(2): 334-341. doi: 10.1007/s12020-012-9662-7
[31]Bain B J. Structure and function of red and white blood cells [J]. Medicine, 2017, 45(4): 187-193. doi: 10.1016/j.mpmed.2017.01.011
[32]Jung K, Ohlrich B, Mildner D, et al. The apoenzyme of aspartate aminotransferase and alanine aminotransferase in the serum of healthy persons and patients suffering from liver diseases [J]. Clinica Chimica Acta, 1978, 90(2): 143-149. doi: 10.1016/0009-8981(78)90515-6
[33] 刘伟, 文华, 蒋明, 等. 饲料蛋白质水平与投喂频率对吉富罗非鱼幼鱼生长及部分生理生化指标的影响 [J]. 水产学报, 2016, 40(5): 751-762.Liu W, Wen H, Jiang M, et al. Effects of dietary protein level and feeding frequency on growth and some physiological-biochemical indexes of GIFT strain of juvenile Nile tilapia (Oreochromis niloticus) [J]. Journal of Fisheries of China, 2016, 40(5): 751-762.
[34] 周顺伍. 动物生物化学 [M]. 第三版. 北京: 中国农业出版社, 1999: 80-129.Zhou S W. Animal Biochemistry [M]. The third edition. Beigjing: China Agricultural Press, 1999: 80-129.
[35]Xu C, Liu W B, Remø S C, et al. Feeding restriction alleviates high carbohydrate diet-induced oxidative stress and inflammation of Megalobrama amblycephala by activating the AMPK-SIRT1 pathway [J]. Fish & Shellfish Immunology, 2019(92): 637-648.
[36]Yang S, Lian G. ROS and diseases: role in metabolism and energy supply [J]. Molecular and Cellular Biochemistry, 2020, 467(1): 1-12.
[37] 李效宇, 刘永定, 宋立荣, 等. 鲤肝细胞抗氧化系统对微囊藻毒素毒性的反应 [J]. 水生生物学报, 2003, 27(5): 472-475. doi: 10.3321/j.issn:1000-3207.2003.05.006Li X Y, Liu Y D, Song L R, et al. Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR [J]. Acta Hydrobiologica Sinica, 2003, 27(5): 472-475. doi: 10.3321/j.issn:1000-3207.2003.05.006
[38]Rodriguez-Dominguez A, Connell S D, Leung J Y S, et al. Adaptive responses of fishes to climate change: Feedback between physiology and behaviour [J]. Science of the Total Environment, 2019(692): 1242-1249.
[39]Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges [J]. Analytical Biochemistry, 2017(524): 13-30.
[40] 姚仕彬, 叶元土, 蔡春芳, 等. 丙二醛对离体草鱼肠道黏膜细胞的损伤作用 [J]. 水生生物学报, 2015, 39(1): 133-141. doi: 10.7541/2015.17Yao S B, Ye Y T, Cai C F, et al. Damage of MDA on intestinal epithelial cells in vitro of grass carp (Ctenopharyngodon idella) [J]. Acta Hydrobiologica Sinica, 2015, 39(1): 133-141. doi: 10.7541/2015.17
[41] 卢德勋. 动物机体自我营养调控功能及其实践意义 [J]. 内蒙古畜牧科学, 1995(1): 1-10.Lu D X. Self-nutrition regulation function of animal body and its practical significance [J]. Inner Mongolia Animal Husbandry Science, 1995(1): 1-10.
[42]Corrales J, Noga E J. Effects of feeding rate on the expression of antimicrobial polypeptides and on susceptibility to Ichthyophthirius multifiliis in hybrid striped (sunshine) bass (Morone saxatilis ♂×M. chrysops ♀) [J]. Aquaculture, 2011, 318(1): 109-121.
相关知识
饲料中添加荷叶提取物对草鱼生长和机体健康的影响
生物发酵饲料对仙居鸡产蛋性能、蛋品质及血清生化指标的影响
如何管理和控制饲料中的水分含量?
利用罗非鱼下脚料提取鱼油的工艺研究
专业饲养员告诉你:为什么说在动物园投喂=投毒?
鱼虾健康高效生长的“密码”——二甲酸钾
茶多酚对蛋鸡生产性能、蛋品质和机体健康的影响
河南师范大学水产饲料配方模型与鱼类营养代谢性疾病
禾丰邵彩梅:欧洲饲用抗生素对我国饲料企业的启示
一种含有银杏叶的饲料饲喂肉鸡的方法.pdf
网址: 饲料能量密度和投喂水平对吉富罗非鱼生长和健康的影响 https://m.trfsz.com/newsview889152.html
