ABSTRACT
Background
The COVID-19–related lockdown has profoundly changed human behaviors and habits, impairing general and psychological well-being. Along with psychosocial consequences, it is possible that sexual behavior was also affected.
Aims
With the present study, we evaluated the impact of the community-wide containment and consequent social distancing on the intrapsychic, relational, and sexual health through standardized psychometric tools.
Methods
A case-control study was performed through a web-based survey and comparing subjects of both genders with (group A, N = 2,608) and without (group B, N = 4,213) sexual activity during lockdown. The Welch and chi-square tests were used to assess differences between groups. Univariate analysis of covariance, logistic regression models, and structural equation modeling were performed to measure influence and mediation effects of sexual activity on psychological, relational, and sexual outcomes.
Outcomes
Main outcome measures were General Anxiety Disorder-7 for anxiety, Patient Health Questionnaire-9 for depression, Dyadic Adjustment Scale for quality of relationship and a set of well-validated sexological inventories (International Index of Erectile Function, Female Sexual Function Index, and male-female versions of the Orgasmometer).
RESULTS
Anxiety and depression scores were significantly lower in subjects sexually active during lockdown. Analysis of covariance identified gender, sexual activity, and living without partner during lockdown as significantly affecting anxiety and depression scores (P < .0001). Logistic regression models showed that lack of sexual activity during lockdown was associated with a significantly higher risk of developing anxiety and depression (OR: 1.32 [95% CI: 1.12 - 1.57, P < .001] and 1.34 [95% CI: 1.15 - 1.57, P < .0001], respectively). Structural equation modeling evidenced the protective role of sexual activity toward psychological distress (βmales = -0.18 and βfemales = -0.14), relational health (βmales = 0.26 and βfemales = 0.29) and sexual health, both directly (βmales = 0.43 and βfemales = 0.31), and indirectly (βmales = 0.13 and βfemales = 0.13).
Clinical translation
The demonstrated mutual influence of sexual health on psychological and relational health could direct the clinical community toward a reinterpretation of the relationship among these factors.
Strengths and limitations
Based on a large number of subjects and well-validated psychometric tools, this study elucidated the protective role of sexual activity for psychological distress, as well for relational and sexual health. Main limitations were the web-based characteristics of the protocol and the retrospective nature of prelockdown data on psychorelational and sexual health of subjects recruited.
CONCLUSIONS
COVID-19 lockdown dramatically impacted on psychological, relational, and sexual health of the population. In this scenario, sexual activity played a protective effect, in both genders, on the quarantine-related plague of anxiety and mood disorders.
Keywords: SARS-Cov-2, COVID-19, Psychological Distress, Sexual Activity, Quarantine, Sexual Health
INTRODUCTION
Since December 2019, the highly transmissible severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing a complex new coronavirus disease named COVID-19, has reached every corner of the planet becoming a global pandemic. Italy has been one of the most severely affected countries, holding the world record of COVID-19 casualties from March to the last weeks of April (>232.000 confirmed cases and >33.000 deaths as of May 29th 2020).1 This forced the Italian government to place the entire country in severe lockdown, a typical community-wide containment intervention used to minimize potential exposure and contagion to a transmissible disease, and designed as a measure of restriction to the movement of citizens, aiming to reduce personal interactions. The success of this ineludible strategy requires cooperation of individuals, as well as personal adherence to the regulations.2 For this reason, the opinion leaders are attempting to implement adherence to the norms of the quarantine not only with law enforcement, but also via adequate communication, reassurance, and practical advice. Although beneficial in terms of health and survival, the experience of confinement is mostly perceived as dramatically unpleasant.3 In many countries, the lockdown was similar to a real quarantine, entailing an extreme limitation of freedom, the impossibility of having physical contacts even with the closest affections, a sense of boredom and helplessness, and a general state of uncertainty about the future. This condition induces the onset of trauma-related distress symptoms, often with dramatic results in the most fragile people such as acute4 and chronic5 stress, sleep disorders,6 anxiety and panic,7 depression,8 adjustment disorder,9 and suicidal ideation.10
Together with several psychological issues characterizing the pandemic,11 it is possible that sexual behavior was also affected during COVID-19 era.12 Male and female sexual function is, in fact, largely dependent on the several biopsychosocial factors that significantly changed during the quarantine.11 Although direct evidence and empirical observations are still lacking, the “#StayAtHome” motto may have dramatically affected intimacy and sexuality.
Research Aims
Considering the dearth of evidence on strategies to increase the individual compliance to the government’s prescription13 and the need to improve the psychological well-being in the quarantine, we aimed to explore the psychological, relational, and sexual health through an Internet survey. Hence, we built an ad hoc website to explore, in Italy during the breakdown, anxiety, depression, dyadic adjustment, sexual function and dysfunction, and their reciprocal relationships by using a set of well-validated psychometric tools. The complete survey, called Sex@COVID, was anonymously administered online starting on April 7th, 2020, until the end of the phase 1 restrictions (May 4th, 2020). Results were also used to explore the mechanisms through which psychological suffering, changes in couple relationships, and social isolation could influence sexual function, and whether gender-based differences could be identified in such regards.
MATERIALS AND METHODS
Study Population and Protocol
For the present study, we calculated a sample size of n = 2,401, based on a target population of ~40 million adults (estimated from the Italian National Institute of Statistics 2019 report on Information and Communication Technologies)14 with a 95% confidence interval and ±2% margin of error.
Given the current lockdown situation, a web-based, anonymous, self-report questionnaire was considered the best strategy to perform the study. Subjects were asked to provide informed consent before starting the survey. In accordance with research aims, a case-control study design was used.
The questionnaire included several demographic information, as well as all necessary psychometric and sexological measures. Participants were asked to provide information concerning whether they were living with or without their partner, if any, during lockdown, and whether they were quarantined because of their symptoms.
The survey was then uploaded to a dedicated website, which was advertised through social media, radio broadcast, and interviews on national newspapers. A total of 7,184 questionnaires were collected. About 363 questionnaires were discarded because of not meeting inclusion criteria (age > 18y, give inform consent and complete each part of the questionnaire). Therefore, 6,821 (females, 4,177; males: 2,644; mean age 32.83 ± 11.24 years) subjects were included in the subsequent analysis and divided in 2 study groups in accordance with being sexually active (eg, engagement in sexual intercourse) during lockdown (Figure 1).
Figure 1.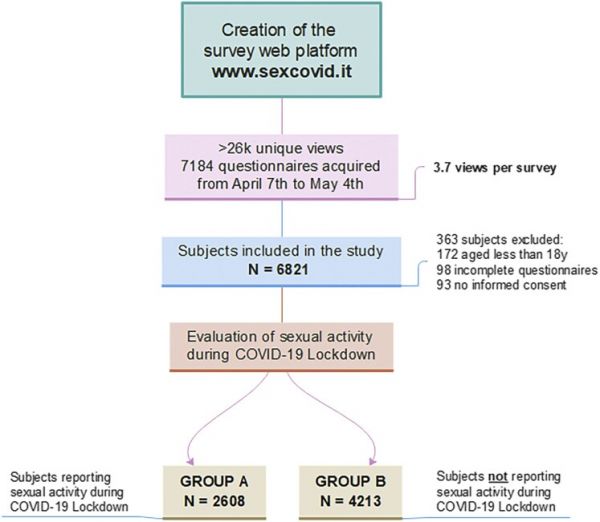
Sex@COVID study flowchart. Flowchart from the creation of the questionnaire web-based platform to the subdivision of the sample in 3 study groups. Criteria for exclusion: (i) age below 18 years; (ii) missing informed consent; (iii) uncompleted questionnaire. Basing on self-referred sexual activity during lockdown, study sample was subdivided in 2 groups: (group A) subjects sexually active during lockdown (N = 2,608); (group B) subjects reported no sexual activity during lockdown (N = 4,213). Figure 1 is available in color online at www.jsm.jsexmed.org.
This research complied with the relevant ethical regulations. Written informed consent was obtained for all participants. Ethical approval was granted by institutional ethics committee.
Measures
Anxiety has been evaluated via the Generalized Anxiety Disorder scale (GAD-7). This self-administered measurement tool consists of 7 items, along a 4-Likert scale, “0” (not at all) to “3” (nearly every day). This scale has been validated for clinical use and allows assessment of the effects of treatments through different levels of cutoff scores. A GAD-7 cutoff score ≥10 was used to determine the presence of general anxiety disorder.15
Depression has been evaluated by the Patient Health Questionnaire (PHQ-9), a self-reported measurement tool composed by 9 items along a 4-Likert scale, “0” (not at all) to “3” (nearly every day), also validated for clinical use. For this study, a cutoff score of ≥10 was used to determine the presence of depressive disorder.16 Both GAD-7 and PHQ-9 have recently been used to assess the psychological burden of COVID-19.17
We assessed the quality of relationship with the Dyadic Adjustment Scale (DAS), a 32-item scale used to investigate relationship adjustment in couples. The DAS has 4 subscales (Dyadic Consensus, Dyadic Satisfaction, Dyadic Cohesion, and Affection Expression) focusing on different aspects of relationship. Subscales can be used independently or together to have an overall score of dyadic adjustment.18,19
Male sexual function was assessed by the International Index of Erectile Dysfunction (IIEF-15), a self-report test composed of 15 items with 6 possible responses, investigating the 5 domains of male sexual function (erectile function, orgasmic function, sexual desire, intercourse satisfaction, and overall satisfaction) over the 4 weeks before compilation. The score of mild erectile dysfunction ranges from 22 to 25, a mild to moderate from 21 to 17, moderate from 16 to 11, and severe from 10 to 6. This test has an adequate explanation of sexological terms regarding the items.20,21
The IIEF-5, or Sexual Health Inventory for Men, an abridged, 5-item version of the IIEF, is often used in the clinical setting, as it provides a quicker, yet solid evaluation of the erectile function of the patient; however, the Sexual Health Inventory for Men does not investigate the remaining subdomains, therefore missing on aspects of male sexuality not necessarily associated with erection, such as desire, ejaculation, and orgasm.22
Finally, the self-perceived orgasmic intensity was assessed by the male Orgasmometer, a single-item Likert scale, derived from Visual Analog Scale for Pain,23 assessing how intense is the perception of the orgasmic experience, ranging from 1 (lowest intensity) to 10 (maximum intensity).24
Female sexual functioning was assessed via the Female Sexual Function Index (FSFI), a self-report test composed of 19 items with 6 possible responses investigating female sexuality in accordance with the following scales or domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. Moreover, it is possible to calculate a total score with a cutoff corresponding to 26.5. Even this test has an adequate explanation of sexological terms into the items. The FSFI investigates with each item the 4 weeks before compilation, therefore depicting the current clinical scenario during lockdown.25,26 In this case, differently from the male subset, the abridged form called FSFI-627 was not used being a screener not useful for the present purpose.
Finally, the quality and intensity of orgasm response was assessed by the female Orgasmometer, a single-item Likert scale, derived from Visual Analog Scale for Pain,23 assessing how intense is the perception of the orgasmic experience, ranging from 1 (lowest intensity) to 10 (maximum intensity).28
Statistical Analysis
Descriptive statistics were performed on the variable of interest; differences between genders were assessed using either independent samples Welch’s t-test or chi-squared test, as appropriate based on the nature of the data. Effect size were performed to evaluate the strength of each statistical analysis and measured with Cohen’s d for Welch’s t-test and Cramer’s V for chi-squared test.
One-way analysis of covariance was performed to assess differences between study groups, based on gender, and sexual activity, work status and cohabitation with the partner during lockdown. Logistic regression models (with Tukey post hoc analysis) were used to evaluate protective and/or potentially deleterious factors related with clinical categories among research variables of interest. Effect size was measured with Cohen’s f for both analysis of covariance and logistic regression models. Analysis was performed by using the statistical software R (version 3.6.3), mainly using the tidyverse, Rmisc car, effectsize and multcomp packages.
To investigate the impact of frequency of sexual activity on study variables, structural equation modeling (SEM) was carried out with Analysis of Moment Structures package for IBM SPSS (version 26.0)29, through which the path diagram was drawn.
3 latent factors were created: an anxiety/depression latent factor (named Psychological Distress, PsyD) was derived from PHQ-9 and GAD-7 scores, a dyadic adjustment latent factor (named Relational Health, RelH) was derived from the cohesion and satisfaction subscales of the DAS-32 and a sexological latent factor (named Sexual Health, SexH) was derived from subscales of male and female sexological inventories (IIEF-15 and Male Orgasmometer for men; FSFI and Female Orgasmometer for women). Latent factors are preferable with respect to manifest variables because they are free from measurement error, and, hence, yield more reliable findings.
The final path models (one for each gender) were appropriately conducted in accordance with modification indices. Standardized regression weights were used to represent path coefficients between variables with P-values below 0.05. The overall fitting model was evaluated with the following indices: ratio of χ2 values and degrees of freedom values (CMIN/DF), goodness-of-fit index (GFI), normed fit index (NFI), standardized root means square residuals (SRMR), and root mean square error of approximation (RMSEA). For each index, the following cutoff values to measure goodness of fit are considered acceptable: for NFI and GFI values equal or greater than 0.90, for SRMR values below 0.08 or 0.05, and for RMSEA values lower than or equal to 0.08. R2 is equal to the variance explained for sexual variables in the model.
Statistical significance was set at P < .05 for all tests.
RESULTS
Descriptive data from study population, grouped in accordance with sexual activity during lockdown, are reported in Table 1 . Not surprisingly, almost half of the study population came from Northern Italy (47.32%), the part of the Country most affected by the epidemic. No significant difference was observed concerning the self-referred prevalence of SARS-CoV-2 positivity between men and women ( = 1.071, P = .3). About 2,608 participants (38.2%) referred to be sexually active during lockdown (group A). On the contrary, 4,213 subjects (61.8%) reported no sexual activity during lockdown (group B): 3,428 (81.4%) were sexually active before the onset of containment measures, whereas 785 (18.6%) did not have any prior sexual activity.
Table 1.
Samples characteristics and univariate comparisons between the sexually active (group A) and sexually inactive (group B) subjects during COVID-19 lockdown
Variable Overall (N = 6,821) Group A (N = 2,608) Group B (N = 4,213) Statistics Effect size (d or V) n (%)/mean ± SD n (%)/mean ± SD n (%)/mean ± SD Age 32.83 ± 11.24 35.94 ± 11.02 30.91 ± 10.93 t = 18.43∗ d = 0.46 Gender Male 2,644 (38.8%) 985 (37.8%) 1,659 (39.4%) = 1.76 Female 4,177 (61.2%) 1,623 (62.2%) 2,554 (60.6%) Geographic area Italy— North 3,228 (47.3%) 1,496 (57.3%) 1732 (41.1%) = 171.67∗ V = 0.16 Italy—Center-South 3,268 (47.9%) 1,003 (38.5%) 2,265 (53.8%) Outside Italy 325 (4.8%) 109 (4.2%) 216 (5.1%) Sexual orientation Heterosexual 5,921 (86.8%) 2,287 (87.7%) 3,634 (86.3%) = 2.90 Not heterosexual 900 (13.2%) 321 (12.3%) 579 (13.7%) Relational status Single 2064 (30.3%) 142 (5.5%) 1922 (45.6%) = 2,897.31∗ V = 0.65 Engaged 2,657 (39.0%) 705 (27.0%) 1952 (46.3%) Cohabitant 1,169 (17.1%) 1,044 (40.0%) 125 (3.0%) Married 931 (13.6%) 717 (27.5%) 214 (5.1%) Education Secondary 2,630 (38.6%) 1,018 (39.0%) 1,612 (38.3%) = 0.40 Graduate 4,191 (61.4%) 1,590 (61.0%) 2,601 (61.7%) Self-referred mood symptoms Yes 964 (14.1%) 353 (13.5%) 611 (14.5%) = 1.24 No 5,857 (85.9%) 2,255 (86.5%) 3,602 (85.5%) Sexually active during 2 months before lockdown Yes 6,036 (88.5%) 2,608 (100%) 3,428 (81.4%) = 549.14∗ V = 0.28 No 785 (11.5%) - 785 (18.6%) Self-referred SARS-COV 2 Positivity Yes 200 (2.9%) 69 (2.6%) 131 (3.1%) = 1.22 No 6,621 (77.1%) 2,539 (97.4%) 4,082(96.9%) Leaving home during breakout Up to 1 a week 5,103 (74.8%) 1822 (69.9%) 3,281 (77.9%) = 54.93∗ V = 0.09 >1 times a week 1,718 (25.2%) 786 (30.1%) 932 (22.1%) Home sharing during breakout Alone 1,035 (15.2%) 168 (6.4%) 867 (20.6%) = 3,293.29∗ V = 0.69 Partner 2,215 (32.5%) 1910 (73.3%) 305 (7.3%) Relatives 2,708 (39.7%) 282 (10.8%) 2,426 (57.5%) Others 863 (12.6%) 248 (9.5%) 615 (14.6) Work status during breakout Unemployed 465 (6.8%) 155 (5.9%) 310 (7.4%) = 83.61∗ V = 0.11 Temporary lay-off 1,549 (22.7%) 549 (21.1%) 1,000 (23.7%) Smart working 3,422 (50.2%) 1,231 (47.2%) 2,191 (52.0%) Office working 877 (12.9%) 410 (15.7%) 467 (11.1%) Both 508 (7.4%) 263 (10.1%) 245 (5.8%)Among the variables of interest, the 2 groups differed significantly in regards to mean age (group A: 35.94 ± 11.02; group B, 30.91 ± 10.93; P < .0001) and relational status, with subjects in group A being prevalently married or cohabitant (69.3%) and subjects in group B being prevalently single or engaged (91.9%) ( = 2,897.31, P < .0001). Similarly, subjects from group A were more likely to be spending lockdown with their partners, whereas participants from group B were mostly living alone or with their relatives ( = 3,293.29, P < .0001). Very interestingly, 26.7% of sexually active people did not spent lockdown with their partner, whereas 7.3% of sexually inactive ones lived with their partners. Despite no significant difference was found among study groups, a consistent amount of the sample (14.1%) reported the presence of psychological symptoms (such as stress, anxiety, and depression) before the lockdown.
Clinical outcomes are presented in Table 2 . A statistically significant difference was observed between the 2 study groups in regard to the raw scores for both the GAD-7 (group A: 6.01 ± 4.23; group B: 7.26 ± 4.44; P < .0001) and PHQ-9 (group A: 6.73 ± 4.75; group B: 8.31 ± 5.17; P < .0001) questionnaires. We initially performed a one-way analysis of covariance model to measure how gender, sexual activity during lockdown, work status due to lockdown measures and living with the partner influenced GAD-7 and PHQ-9 scores. These findings are summarized in Figure 2 and reported in detail in Supplementary Table 1. Higher GAD-7 scores were found for women (β = 2.33, SE = 0.20, P < .001), subjects reporting no sexual activity during lockdown (β = 0.89, SE = 0.39, P < .05), and those separated from their partner (β = 1.00, SE = 0.30, P < .001); similarly, higher PHQ-9 scores were found for women (β = 2.28, SE = 0.23, P < .001), subjects reporting no sexual activity during lockdown (β = 0.94, SE = 0.45, P < .05), and those separated from their partner (β = 1.31, SE = 0.35, P < .001). Although both models were significant (P < .0001), they explained a weak proportion of variance (adjusted R2 0.08 and 0.07 for GAD-7 and PHQ-9 scores, respectively) and no significant interaction between gender, sexual activity during lockdown, and living with the partner was found.
Table 2.
Clinical outcomes and univariate comparisons between the sexually active (group A) and sexually inactive (group B) subjects during COVID-19 lockdown
Variable Overall (N = 6,821) Group A (N = 2,608) Group B (N = 4,213) Statistics Effect size (d or V) n (%)/mean ± SD n (%)/mean ± SD n (%)/mean ± SD Anxiety GAD-7 score 6.78 ± 4.40 6.01 ± 4.23 7.26 ± 4.44 t = −11.626∗ d = 0.29 Prevalence (%) 1,624 (23.8%) 478 (18.3%) 1,146 (27.2%) = 69.92∗ V = 0.10 Depression PHQ-9 score 7.70 ± 5.07 6.73 ± 4.75 8.31 ± 5.17 t = −12.898∗ d = 0.32 Prevalence (%) 2,045 (30.0%) 596 (22.9%) 1,449 (34.4%) = 102.20∗ V = 0.12 Dyadic adjustment Cohesion subscale 15.86 ± 3.93∗ 16.12 ± 3.84† 15.58 ± 4.00‡ t = 4.781∗ d = 0.14 Satisfaction subscale 36.57 ± 7.42∗ 37.00 ± 7.11† 36.11 ± 7.72‡ t = 4.113∗ d = 0.12Figure 2.
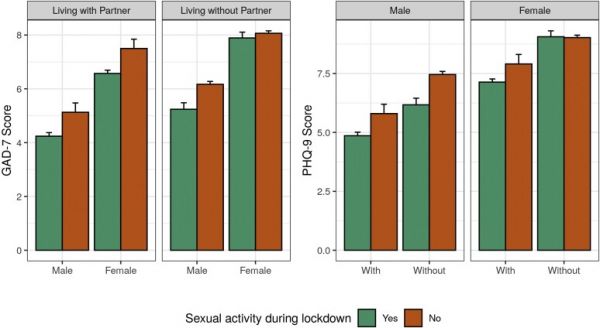
Sexually active subjects during lockdown show lower anxiety and depressive symptoms. Factorial analysis of covariance was computed to evaluate differences in anxiety (F(7, 6,813) = 86.69, P < .0001, Cohen’s F [95% CI] = 0.24 [0.22, 0.27], 0.15 [0.12, 0.17] and 0.08 [0.06, 0.11] for gender, sexual activity during lockdown and living without partner during lockdown, respectively) and depression (F(7, 6,813) = 73.71, P < .0001, Cohen’s F [95% CI] = 0.19 [0.17, 0.21], 0.16 [0.14, 0.18], and 0.11 [0.09, 0.13] for gender, sexual activity during lockdown and living without partner during lockdown, respectively) scores among subjects sexually active during lockdown (group A) and subjects reported no sexual activity during lockdown (group B). Data expressed as means ± SE; detailed outputs of the models, including coefficients and effect sizes for interaction terms, are available in Supplementary Table 1. GAD = Generalized Anxiety Disorder scale; PHQ = Patient Health Questionnaire. Figure 2 is available in color online at www.jsm.jsexmed.org.
Based on these premises, we decided to perform logistic regression analysis to identify the effects of different variables on the prevalence of anxiety and depression. As stated in the methods, presence of anxiety and disorder in the study population was measured in accordance with GAD-7 and PHQ-9 scores, using a cutoff score ≥10 for both. As depicted in Figure 3, female gender, lack of sexual activity exclusively during lockdown, living without partner during lockdown, age greater than 40 years, self-referred psychological symptoms before the lockdown, being temporary lay-off and unemployed were all significantly associated with an increased risk of developing anxiety and depression. When addressing the effects of sexual activity on both anxiety and depression, no significant effect was found comparing people who were sexually active during lockdown to those who never had any prior sexual activity; on the other hand, when comparing sexually active subjects to those who did not have sexual activity during lockdown, a significantly higher risk of developing anxiety and depression was found (OR 1.32 [95% CI: 1.12 - 1.57] and 1.34 [95% CI: 1.15 - 1.57], respectively). Regression coefficients, their standard errors and the resulting odds ratios and confidence intervals are reported in detail in Supplementary Table 2.
Figure 3.
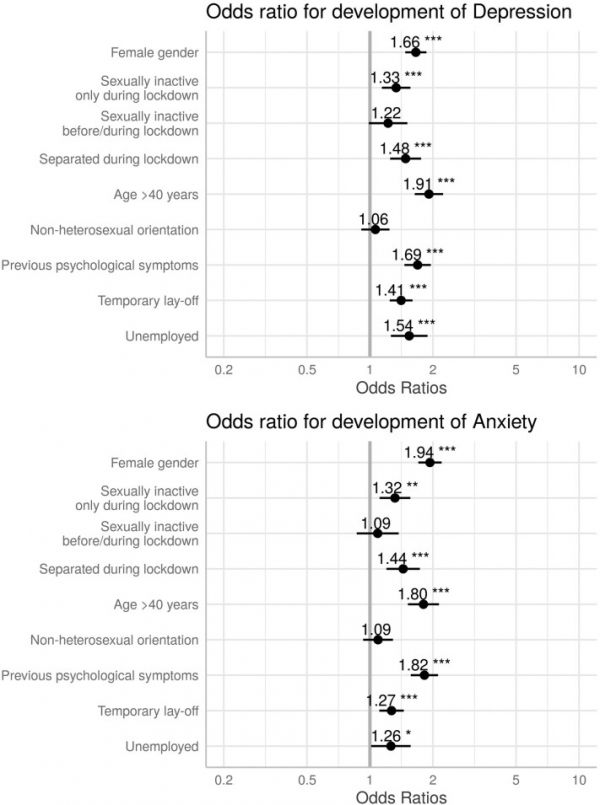
Odds ratio for anxiety and depression in the study population. Results from logistic regression models identify, among other variables, that cessation of sexual activity during lockdown is associated with higher risk of developing both anxiety and depression (OR 1.32 [95% CI: 1.12–1.57, P = .0035] and 1.34 [95% CI: 1.15–1.57, P = .0001], respectively). Full outputs for both models are reported in Supplementary Table 2.
Analysis of the relationship quality, measured with the DAS, showed that, as expected, group B had lower scores than group A (P < .0001) in all dyadic domains (cohesion: 15.58 ± 4.00 vs 16.12 ± 3,84, respectively; satisfaction: 36.11 ± 7.72 vs 37.00 ± 7.11, respectively).
Sexual outcomes, limited to group A, are presented in Table 3 . Subjects younger than 40 years reported a higher frequency of sexual activity during lockdown, compared with subjects older than 40 years. Conversely, no statistical differences were observed in the comparison of sexological inventories in both sexes subdivided on the age cutoff value of 40, except for desire (4.16 ± 1.16 vs 3.96 ± 1.20) and coital pain (5.45 ± 0.96 vs 5.31 ± 1.06) domains of the FSFI, where younger women report better scores.
Table 3.
Sexological outcomes and univariate comparisons between the subjects under and over age of 40
Variable Group A <40 years >40 years Statistics Effect size (d or V) n (%)/mean ± SD n (%)/mean ± SD n (%)/mean ± SD Sexual activity during lockdown N = 2,608 N = 1862 N = 746 Up to 1 a week 1,504 (57.7%) 1,021 (54.8%) 483 (64.7%) = 21.43∗∗ V = 0.09 More than 1 a week 1,104 (42.3%) 841 (45.2%) 263 (35.3%) Male sexual function N = 985 N = 528 N = 457 Desire 8.16 ± 1.63 8.19 ± 1.67 8.12 ± 1.58 t = 0.666 Erectile function 27.41 ± 4.11 27.65 ± 3.96 27.13 ± 4.26 t = 1.925 Orgasm 9.12 ± 1.61 9.11 ± 1.66 9.14 ± 1.56 t = −0.214 Intercourse satisfaction 11.62 ± 2.70 11.70 ± 2.67 11.52 ± 2.73 t = 1.054 Overall satisfaction 7.31 ± 2.22 7.40 ± 2.28 7.22 ± 2.15 t = 1.246 SHIM score 23.11 ± 3.60 23.22 ± 3.58 22.98 ± 3.64 t = 1.075 ED prevalence (%) 182 (18.5%) 86 (16.3%) 96 (21.0%) χ21 = 3.62 Orgasmometer 7.38 ± 1.53 7.46 ± 1.56 t = −0.714 Female sexual function N = 1,623 N = 1,334 N = 289 Desire 4.12 ± 1.17 4.16 ± 1.16 3.96 ± 1.20 t = 2.550∗ d = 0.17 Arousal 4.66 ± 1.20 4.68 ± 1.17 4.58 ± 1.33 t = 1.362 Lubrication 5.23 ± 1.05 5.25 ± 1.03 5.15 ± 1.13 t = −1.402 Orgasm 4.70 ± 1.36 4.69 ± 1.34 4.76 ± 1.41 t = −0.823 Sexual satisfaction 4.64 ± 1.31 4.62 ± 1.31 4.72 ± 1.31 t = −1.184 Coital pain 5.33 ± 1.04 5.45 ± 0.96 5.31 ± 1.06 t = 2.178∗ d = 0.14 FSFI total score 28.68 ± 5.38 28.84 ± 5.24 28.48 ± 5.99 t = −0.210 FSD prevalence (%) 467 (28.8%) 380 (28.5%) 87 (30.1%) χ21 = 0.30 Orgasmometer 7.08 ± 1.84 7.12 ± 2.22 t = −0.326To assess the risk of multicollinearity or sphericity, correlation analysis to assess the relationship between variables was performed. Results of correlation analysis are represented in Figure 4 . Because no correlation was deemed able to significantly affect the regression models, all variables were included in all subsequent steps of analysis.
Figure 4.
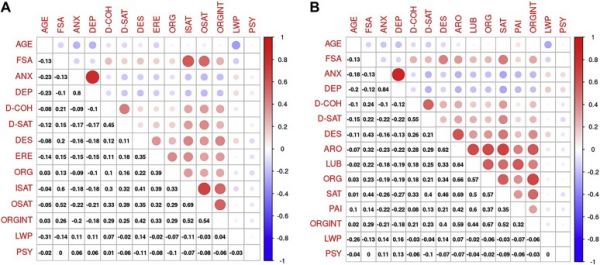
Correlation matrices for variables included in structural equation model (SEM). Correlation plots depicting the measure of correlation between variables included in SEM. Spearman’s correlation coefficients are given in the lower triangle, whereas colored circles indicate statistically significant correlations in the upper triangle. Red represents positive and blue negative correlations, while size and color intensity of the circles are related to the correlation coefficients. (A) Male sexuality: Frequency of sexual activity (FSA) is weakly correlated with GAD-7 (ANX) and PHQ-9 (DEP) scores and with sexual domains (from weakest to strongest): Orgasm (ORG), Erectile function (ERE), Desire (DES), Male Orgasmometer (ORGINT), Overall Satisfaction (OSAT) and Intercourse Satisfaction (ISAT). Moreover, FSA is weakly correlated with dyadic satisfaction (DAS-SAT) and moderately with dyadic cohesion (DAS-COH). (B) Female sexuality: Frequency of sexual activity (FSA) is weakly correlated with GAD-7 (ANX) and PHQ-9 (DEP) scores and with sexual domains (from weakest to strongest): Coital Pain (PAI), Lubrication (LUB), Orgasm (ORG), and Arousal (ARO), Female Orgasmometer, (ORGINT), Desire (DES) and Sexual Satisfaction (SAT). Moreover, FSA is moderately correlated with dyadic cohesion (DAS-COH) and dyadic satisfaction (DAS-SAT). Figure 4 is available in color online at www.jsm.jsexmed.org.
To assess the relationship between frequency of sexual activity during lockdown with psychological symptoms, relational quality, and sexual function, we performed maximum likelihood SEMs, separately, for both genders (Figure 5) belonging to group A. We used age, previous psychological symptoms, and living without the partner during lockdown as covariates, frequency of sexual activity (FSA, ranging from 1 = less than 1 time a week to 5 = more than 1 time a day) as exogenous variable, with psychological distress (PsyD, composed by anxiety/depression score of GAD-7 and PHQ-9, respectively) and relational health (RelH, composed by dyadic cohesion and satisfaction subscales of the DAS) as mediator variables, and female/male sexual health (SexH, resulting from scores of each sexual domains of IIEF and FSFI and Orgasmometer) as latent dependent (outcome) variable. PsyD has a direct negative effect on SexH, irrespective of gender (β = -0.23, P < .0001 in men; β = -0.21, P < .0001 in women). Conversely, RelH has a direct positive effect on SexH (β = -0.33, P < .0001 in men; β = -0.34, P < .0001 in women). FSA significantly mediates, in a protective way, levels of PsyD (β = -0.18, P < .0001 in men; β = -0.14, P < .0001 in women), RelH (β = 0.26, P < .0001 in men; β = 0.29, P < .0001 in women) and SexH (β = 0.43, P < .0001 in men; β = 0.31, P < .0001 in women). The amounts of variance explained (R2) in the models are 49% and 33% for male and female sexual health, respectively. GFIs of SEMs were acceptable (males: χ2/df = 6.18; SRMR = 0.050; NFI = 0.923; GFI = 0.953; RMSEA = 0.073; females: χ2/df = 7,878; SRMR = 0.049; NFI = 0.944; GFI = 0.958; RMSEA = 0.065).
Figure 5.
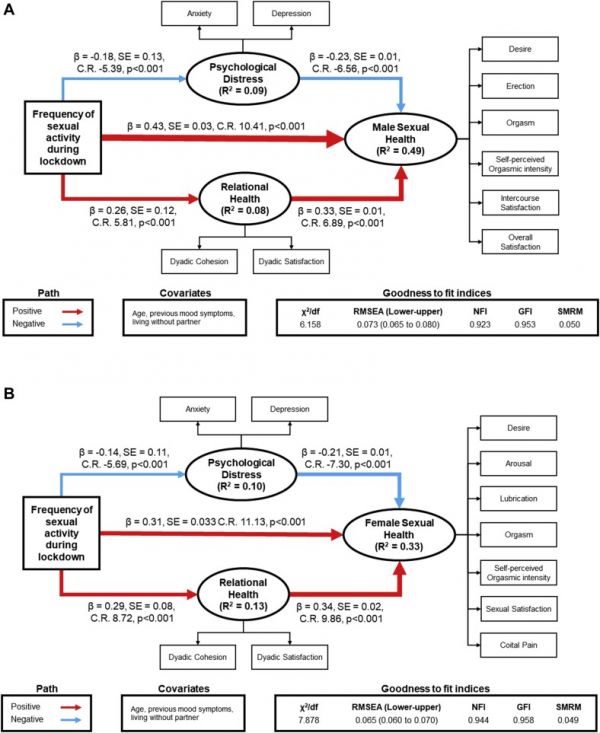
Frequency of sexual activity is related to lower psychological distress and better relational and sexual health. Red arrows indicate positive paths, whereas blue ones indicate negative paths. Arrow widths are scaled to reflect the magnitude of path coefficients. Structural equation model showing that frequency of sexual activity is associated with both lower anxiety and low depressive symptoms (expressed in the psychological distress latent variable) and higher levels of dyadic cohesion and satisfaction (expressed in the relational health latent variable) in both genders. Frequency of sexual activity is also associated with better female and male sexual function and self-perception of orgasmic intensity (expressed in the sexual health latent variable), both in direct and indirect way. (A) Graphical representation of the structural equation model for the male gender. (B) Graphical representation of the structural equation model for the female gender. Figure 5 is available in color online at www.jsm.jsexmed.org.
Regarding indirect effects, FSA describes the 23% and 29% of total effect mediated in men and women, respectively. Moreover, we observed that the “FSA=>PsyD=>SexH” and the “FSA=>RelH=>SexH” paths have positive values in men (β = 0.041, P < .001, 7% of total effect mediated and β = 0.089, P < .001, 16% of total effect mediated, respectively) and women (β = 0.030, P < .001, 7% of total effect mediated and β = 0.097, P < .001, 22% of total effect mediated, respectively) (Table 4).
Table 4.
Direct, indirect, and total effects on sexual outcomes
Variable Predictor Direct effect Indirect effect Total effect % Of total effect mediated Male sexual healthR2 = 0.49 FSA 0.435∗ 0.130∗ 0.565∗ 23.0% PsyD −0.339∗ - −0.339∗ RelH 0.238∗ - 0.238∗ Female sexual health
R2 = 0.33 FSA 0.308∗ 0.127∗ 0.435∗ 29.0% PsyD −0.206∗ - −0.206∗ RelH 0.342∗ - 0.342∗
DISCUSSION
The COVID-19 pandemic and the consequent lockdown have had unprecedented, dramatic repercussions at both macrosocial, such as the economy and policy, and microsocial level, such as on the psychological and relational well-being of persons.3,30 This affects not only infected patients, or suspected ones, but also caregivers, health care workers, and quarantined family members.31–33 Moreover, after social isolation, several aspects of daily life have dramatically changed. We found in our naturalistic observation that sexual functioning acts as a predictor and marker of psychological well-being.
If data have been produced on the repercussions of COVID-19–related social isolation norms on the psychological health, and specifically on the prevalence and nature of psychopathological symptoms,13,34 we trust that no studies have so far investigated the sexual health as a variable to evaluate psychological distress due to the COVID-19–related confinement and social isolation.
We found that half of our study sample (50.3%) reported an interruption of sexual activity during lockdown. This demonstrates that the lockdown itself dramatically affects sexual health, for 2 possible reasons: distress due to quarantine and impossibility to reach the preferred sexual partner. Moreover, another COVID-related study highlighted how social distancing due to lockdown negatively impacts on sexual activity.35
We discovered that subjects who could maintain sexual activity during lockdown had lower psychological distress, as proven by both GAD-7 and PHQ-9 scores, than those who had to give up on sexual activity due to lockdown policies (eg, couples separated during lockdown). Moreover, the same scenario is present on a relational level, with subjects who continued to have sexual intercourses during lockdown showing better scores on dyadic cohesion and satisfaction subscales of DAS-32, confirming the evidence that a regular sexual activity leads to a better relational health.36
Not differently from other clinical sets, female gender is more likely to develop anxiety and mood disorders.37,38 Our data agree with evidence regarding both the worsening of the psychological well-being during COVID-19–related social isolation39,40 and the historical knowledge about the major female susceptibility to the development of emotional diseases39 and sexual dysfunctions.41,42 Moreover, together with biological factors43–45 the exposition to a major stressor (pregnancy complications, lack of partner or of social support, history of sexual abuse, presence of life adverse events) increases in women the risk to develop a psychopathology.46 The higher ability of women in reading their own emotional status and that of the others47 may potentially expose them to a peculiar perception of their own positive/negative emotions, and hence to a higher risk to develop psychopathological symptoms. Vice versa, also during the COVID-19 breakdown, men resulted less at risk for the development of symptoms of anxiety and depression.11 Unsurprisingly, we also found that work status like being temporary lay-off from work or being unemployed increased the chance to develop anxiety and depressive symptoms. Lockdown measures had certainly lead a great amount of companies (eg, restaurants, event planners, home-builders, etc.) to stop their activities, with a forced downsizing of their staffs and having recourse to social safety net or also stopping any possible hiring campaigns. This scenario could have a negative impact on psychological status, as confirmed by literature.36,48–50
We found that general sexual functioning of females and males during quarantine was mediated by different variables. If the social isolation, together with the activity restriction and the reduction of rewarding events, may represent a risk factor for the development of a psychopathology and consequent psychorelational difficulties, our data have revealed a novelty in the relationship between sexuality and psychological distress, that is, the role of the frequency of sexual activities on anxiety, depression, couple relationship, and sexual function itself. In other words, we found both more anxious-depressive symptomatology both more sexual dysfunctions in people with lower frequency of sexual activities. At the same time, subjects with higher frequency of intercourse had better sexual functioning, as well as a better dyadic adjustment. Adequate dyadic cohesion and satisfaction represent another protective and positive factor to the safeguard of sexual functioning, in accordance with our findings.
Further proofs of the protective effects of sexual health on anxiety and depression come from our structural equation models, where both anxiety and depression did not negatively mediate the effect of the frequency of sexual activity on sexual health, as shown by the analysis of indirect effects on female and male sexuality. This further strengthens our study evidence that a regular to high sexual activity could decrease the negative effects of anxiety and depressive symptoms toward sexual function, in both genders.
Although the role of anxiety in dramatically affecting sexual performance is clearly recognized,51,52 the anxiolytic effect of successful sexual activity is less studied. Neurobiology of anxiety has found to be complex, involving both centrally and peripherally the GABAergic/opiatergic circuitries and the adrenergic activation, accounting, respectively, for the reduction in the sexual desire and the impairment in arousal and even orgasm.53 More efforts should be paid in the future in exploring how sexual activity, most probably throughout the dopaminergic circuits,54 may directly or indirectly reduce the levels of anxiety.
The positive and healthy effects of an adequate sexual activity on psychological wellness and on relational and sexual health have been documented.55,56 Sexual activity itself is able to trigger not only the activation of the hypothalamus–pituitary–gonadal axis, but also other psycho-neuro-endocrinological factors regulating psycho-sexological fitness.55,57–59 Such a possible mechanism, based on the well-known ability of sexual intercourse to boost testosterone levels, or to maintain optimal androgenic tone,60,61 may explain the negative correlation between sexuality and affective disorders, at least for depression. This has been hypothesized as a bona fide hypothalamic action on GnRH pulsatility, peripherally measured by the LH bioassay as a surrogate marker of LH glycosylation, affecting the ability of the testicular Leydig cell producing testosterone.56,62 As occurring for the physiological reduction of metabolism during forced starvation, the impossibility of regular sexual activity because of the lockdown, or other internal factors, may reset the hypothalamic pulse generator to a lower activity.63 The decreased intercourse frequency has been, in fact, bidirectionally coupled to poor relational health, being its deterioration associated with impairment in sexual activities, and, as here hypothesized, to an evident reduction in testosterone levels.64 Because low levels of testosterone have been related to mood disorders, while reaching eugonadism to a restoration of them,55 the lower presence of depression in male and in both sexes when indulging with the sexual rewards during the COVID-19 quarantine could be hypothesized, at least partially, to be androgen-dependent.63
Interestingly, we found in our study sample that sexual dysfunctions were not age-dependent: this constitutes a unique and peculiar finding. Robust, epidemiological studies unanimously evidence that presence and severity of the very large majority of sexual dysfunctions is directly correlated to age in both sexes.65–67 For example, across epidemiological studies, increasing age appears to be a strong risk factor for erectile dysfunction,68,69 with a prevalence overtly age-dependent, with a steep increase beyond the 5th decade.9 Similarly, epidemiological studies for female sexual disorders reveal that the prevalence, which ranges from 19% to 45%,27 is also highly dependent on biologic, as well as contextual and relationship variables, but ultimately increases with age.70 The evidence that during the COVID-19 lockdown age loses its weight as a statistically significant predictor for sexual dysfunctions might shed a light on the pattern through which psychological suffering impacted on psychological health and, consequently, on sexual health. Indeed, being the COVID-19 pandemic and the lockdown per se conditions that, irrespective of age, brought about new and significant changes in everyone’s daily life habits (including romantic and sexual intimacy), it is tenable to sustain that the prevalence of the sexual dysfunctions in our study sample may reflect this unprecedented scenario.
Differently from sexual dysfunctions, psychological distress—measured in terms of anxiety and depression—resulted significantly poorer in young persons with respect to the older ages. Although these results again do not completely follow the common age distribution, they mirror recent findings related to COVID-19 quarantine in different Italian and Chinese populations.40,71,72 Indeed, it is recognized that in the general population, younger people are more at risk for psychological disorders. This is true especially for anxiety but not for depression. Moreover, psychological status is strictly related to sexual health and depression, and anxiety is a well-known determining factor of overall sexual functioning.73,74
Based on our findings, loneliness during lockdown and the absence of the partner seem to be additional risk factors for the development of symptoms of anxiety and depression, especially in women. This variable partly also influences the frequency of sexual intercourses. If this last aspect is easily explained by the separation from the partner, the increased risk to be prone to anxiety and/or depression due to the solitude is less understandable, especially in younger people, such as those enrolled in this study. In agreement with our findings, literature data suggest a higher vulnerability in a sizeable part of the population to develop psychopathology, if exposed to loneliness.75–78 Hence, we may conclude that the COVID-19–related quarantine has induced a general vulnerability, not only from a general health point of view, but also from a psychological and psychosexological ones.
The COVID-19 pandemic and the consequent lockdown has made possible the measurement of the psychorelational and sexological modifications of the persons in a unique and peculiar social environment. We believe that the present study represents a first, large-scale attempt to explain the modifications of the psychological, relational, and sexological functioning of the individuals exposed to a major social and personal distress. Considering the ability of relational and sexual health in improving intrapsychic health, the former should be carefully considered when establishing the norms of quarantine and when analyzing their efficacy based on the personal adhesion. If sexuality has a major reward role,79,80 it may also have a major motivational role in challenging and difficult tasks. Our findings support the idea to consider relational-sexual health as a fundamental tool to improve adhesion and as a unique predictor of intrapsychic health.
Limitations
The real-life nature of our study produced some limitations, such as the impossibility to have quantitative data about the psychorelational and sexological functioning before the COVID-19 lockdown. Among descriptive data, we found that almost a half of the sample came from northern Italy. This may represent a relative bias of selection, being the population most severely reached by the COVID-19, probably, more interested in participating. Another limitation is related to the use of online investigation for the information collection. If it is true that online surveys have been considered an equal good methodology of the sex surveys for the subjects’ recruitment and the study of specific topics,81 it is even more true that in this specific historical period, characterized by social isolation, online experimental protocols represent the unique possibility to study human behavior.
CONCLUSION
Behavioral sciences play a crucial role in fighting general crises such as the pandemics.82,83 We demonstrated by well-validated tools that the COVID-19 lockdown dramatically impacted on the sexual health of the population. We also found sexual activity as protective, in both genders, to the quarantine-related plague of anxiety, depression and relational issues. Addressing sexual health of the population is proposed, finally, as a pivotal strategy to improve the adhesion to the difficult social norms characterizing the breakdown.
STATEMENT OF AUTHORSHIP
Daniele Mollaioli, Conceptualization, Data curation, Formal Analysis, Supervision, Writing - original draft, Writing - review & editing; Andrea Sansone, Conceptualization, Data curation, Formal Analysis, Supervision, Writing - original draft, Writing - review & editing; Giacomo Ciocca, Conceptualization, Writing - original draft, Writing - review & editing; Erika Limoncin, Conceptualization, Writing - original draft, Writing - review & editing; Elena Colonnello, Conceptualization, Writing - original draft, Writing - review & editing; Giorgio Di Lorenzo,Conceptualization, Supervision, Writing - review & editing; Emmanuele A. Jannini, Conceptualization, Data curation, Supervision, Writing - original draft, Writing - review & editing.
Funding
None.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsxm.2020.10.008.
Supplementary Material
Supplementary table 1
Supplementary table 2
Contributor Information
Daniele Mollaioli, Chair of Endocrinology and Medical Sexology (ENDOSEX), Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Andrea Sansone, Chair of Endocrinology and Medical Sexology (ENDOSEX), Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Giacomo Ciocca, Department of Dynamic and Clinical Psychology, Sapienza University of Rome, Rome, Italy.
Erika Limoncin, Chair of Endocrinology and Medical Sexology (ENDOSEX), Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Elena Colonnello, Chair of Endocrinology and Medical Sexology (ENDOSEX), Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Giorgio Di Lorenzo, Chair of Psychiatry, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Emmanuele A. Jannini, Chair of Endocrinology and Medical Sexology (ENDOSEX), Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
REFERENCES
1. Istituto Superiore di Sanità . COVID-19 epidemic. 14 May 2020 national update. Roma: Istituto Superiore di Sanità, 2020. [Google Scholar] 2. Sinha A. King Lear Under COVID-19 Lockdown. JAMA 2020;323: 1758–1759. [DOI] [PubMed] [Google Scholar] 3. Brooks S.K., Webster R.K., Smith L.E.et al. . The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020;395: 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar] 4. Bai Y., Lin C.C., Lin C.Y.et al. . Survey of stress reactions among health care workers involved with the SARS outbreak. Psychiatr Serv 2004;55: 1055–1057. [DOI] [PubMed] [Google Scholar] 5. DiGiovanni C., Conley J., Chiu D.et al. . Factors influencing compliance with quarantine in Toronto during the 2003 SARS outbreak. Biosecur Bioterror 2004;2: 265–272. [DOI] [PubMed] [Google Scholar] 6. Lee S., Chan L.Y., Chau A.M.et al. . The experience of SARS-related stigma at Amoy Gardens. Soc Sci Med 2005;61: 2038–2046. [DOI] [PMC free article] [PubMed] [Google Scholar] 7. Tucci V., Moukaddam N., Meadows J.et al. . The Forgotten Plague: Psychiatric Manifestations of Ebola, Zika, and Emerging Infectious Diseases. J Glob Infect Dis 2017;9: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar] 8. Hawryluck L., Gold W.L., Robinson S.et al. . SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis 2004;10: 1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar] 9. Cao W., Fang Z., Hou G.et al. . The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Res 2020;287: 112934. [DOI] [PMC free article] [PubMed] [Google Scholar] 10. Reger M.A., Stanley I.H., Joiner T.E.. Suicide Mortality and Coronavirus Disease 2019-A Perfect Storm? JAMA Psychiatry 2020;77: 1093–1094. [DOI] [PubMed] [Google Scholar] 11. Moccia L., Janiri D., Pepe M.et al. . Affective temperament, attachment style, and the psychological impact of the COVID-19 outbreak: an early report on the Italian general population. Brain Behav Immun 2020;87: 75–79. [DOI] [PMC free article] [PubMed] [Google Scholar] 12. Sansone A., Mollaioli D., Ciocca G.et al. . Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J endocrinological Invest 2020. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar] 13. Bavel J.J.V., Baicker K., Boggio P.S.et al. . Using social and behavioural science to support COVID-19 pandemic response. Nat Hum Behav 2020;4: 460–471. [DOI] [PubMed] [Google Scholar] 14. Istituto Nazionale di Statistica . Cittadini e ICT. ISTAT, 2019. Accessed https://www.istat.it/it/files/2019/12/Cittadini-e-ICT-2019.pdf. December 18, 2019. [Google Scholar] 15. Lowe B., Decker O., Muller S.et al. . Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care 2008;46: 266–274. [DOI] [PubMed] [Google Scholar] 16. Kroenke K., Spitzer R.L., Williams J.B.. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar] 17. Lai J., Ma S., Wang Y.et al. . Factors associated with mental health outcomes among health care workers exposed to Coronavirus Disease 2019. JAMA Netw Open 2020;3: [DOI] [PMC free article] [PubMed] [Google Scholar] 18. Spanier G.B. Measuring dyadic adjustment: New scales for assessing the quality of marriage and similar dyads. J Marriage Fam 1976;38: [Google Scholar] 19. Garbarini C., Gerino E., Marino E.et al. . Psychometrical Properties of the Dyadic Adjustment Scale for Measurement of Marital Quality with Italian Couples. Proced - Social Behav Sci 2014;127: 499–503. [Google Scholar] 20. Rosen R.C., Riley A., Wagner G.et al. . The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49: 822–830. [DOI] [PubMed] [Google Scholar] 21. Corona G., Jannini E.A., Maggi M.. Inventories for male and female sexual dysfunctions. Int J Impot Res 2006;18: 236–250. [DOI] [PubMed] [Google Scholar] 22. Rosen R.C., Cappelleri J.C., Smith M.D.et al. . Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11: 319–326. [DOI] [PubMed] [Google Scholar] 23. Joyce C.R.B., Zutshi D.W., Hrubes V.et al. . Comparison of fixed interval and visual analogue scales for rating chronic pain. Eur J Clin Pharmacol 1975;8: 415–420. [DOI] [PubMed] [Google Scholar] 24. Limoncin E., Lotti F., Rossi M.et al. . The impact of premature ejaculation on the subjective perception of orgasmic intensity: validation and standardisation of the ‘Orgasmometer. Andrology 2016;4: 921–926. [DOI] [PubMed] [Google Scholar] 25. Rosen R., Brown C., Heiman J.et al. . The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26: 191–208. [DOI] [PubMed] [Google Scholar] 26. Nappi R.E., Albani F., Vaccaro P.et al. . Use of the Italian translation of the Female Sexual Function Index (FSFI) in routine gynecological practice. Gynecol Endocrinol 2008;24: 214–219. [DOI] [PubMed] [Google Scholar] 27. Isidori A.M., Pozza C., Esposito K.et al. . Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med 2010;7: 1139–1146. [DOI] [PubMed] [Google Scholar] 28. Mollaioli D., Di Sante S., Limoncin E.et al. . Validation of a Visual Analogue Scale to measure the subjective perception of orgasmic intensity in females: The Orgasmometer-F. PLoS One 2018;13: e0202076. [DOI] [PMC free article] [PubMed] [Google Scholar] 29. Arbuckle J.L. Amos (Version 26.0) Computer Program. 26 edn.. Chicago: IBM SPSS, 2019. [Google Scholar] 30. Huang X., Wei F., Hu L.et al. . Epidemiology and Clinical Characteristics of COVID-19. Arch Iran Med 2020;23: 268–271. [DOI] [PubMed] [Google Scholar] 31. Mazza C., Ricci E., Biondi S.et al. . A Nationwide Survey of Psychological Distress among Italian People during the COVID-19 Pandemic: Immediate Psychological Responses and Associated Factors. Int J Environ Res Public Health 2020;17: [DOI] [PMC free article] [PubMed] [Google Scholar] 32. Kisely S., Warren N., McMahon L.et al. . Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ 2020;369: m1642. [DOI] [PMC free article] [PubMed] [Google Scholar] 33. Losada-Baltar A., Jimenez-Gonzalo L., Gallego-Alberto L.et al. . “We’re staying at home”. Association of self-perceptions of aging, personal and family resources and loneliness with psychological distress during the lock-down period of COVID-19. J Gerontol B Psychol Sci Soc Sci 2020. 10.1093/geronb/gbaa048[E-pub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar] 34. West R., Michie S., Rubin G.J.et al. . Applying principles of behaviour change to reduce SARS-CoV-2 transmission. Nat Hum Behav 2020;4: 451–459. [DOI] [PubMed] [Google Scholar] 35. Jacob L., Smith L., Butler L.et al. . COVID-19 Social Distancing and Sexual Activity in a Sample of the British Public. J Sex Med 2020;17: 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar] 36. Álvaro J.L., Garrido A., Pereira C.R.et al. . Unemployment, Self-esteem, and Depression: Differences between Men and Women. The Spanish J Psychol 2019;22: E1. [DOI] [PubMed] [Google Scholar] 37. Altemus M., Sarvaiya N., Neill Epperson C.. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinology 2014;35: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar] 38. Stevens A.W.M.M., Goossens P.J.J., Knoppert-van der Klein E.A.M.et al. . Risk of recurrence of mood disorders during pregnancy and the impact of medication: A systematic review. J Affective Disord 2019;249: 96–103. [DOI] [PubMed] [Google Scholar] 39. Hartung C.M., Lefler E.K.. Sex and gender in psychopathology: DSM-5 and beyond. Psychol Bull 2019;145: 390–409. [DOI] [PubMed] [Google Scholar] 40. Rossi R., Socci V., Talevi D.et al. . COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. An N=18147 web-based survey. medRxiv 2020;11: 790. [DOI] [PMC free article] [PubMed] [Google Scholar] 41. Carosa E., Sansone A., Jannini E.A.. MANAGEMENT OF ENDOCRINE DISEASE: Female sexual dysfunction for the endocrinologist. Eur J Endocrinol 2020;182: R101. [DOI] [PubMed] [Google Scholar] 42. Zheng J., Skiba M.A., Bell R.J.et al. . The prevalence of sexual dysfunctions and sexually related distress in young women: a cross-sectional survey. Fertil Steril 2020;113: 426–434. [DOI] [PubMed] [Google Scholar] 43. Soares C.N. Depression and menopause: An update on current knowledge and clinical management for this Critical Window. Med Clin North Am 2019;103: 651–667. [DOI] [PubMed] [Google Scholar] 44. Allshouse A., Pavlovic J., Santoro N.. Menstrual Cycle Hormone Changes Associated with Reproductive Aging and How They May Relate to Symptoms. Obstet Gynecol Clin 2018;45: 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar] 45. Li S.H., Lloyd A.R., Graham B.M.. Physical and mental fatigue across the menstrual cycle in women with and without generalised anxiety disorder. Horm Behav 2020;118: 104667. [DOI] [PubMed] [Google Scholar] 46. Biaggi A., Conroy S., Pawlby S.et al. . Identifying the women at risk of antenatal anxiety and depression: A systematic review. J Affect Disord 2016;191: 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar] 47. Baron-Cohen S., Bowen D.C., Holt R.J.et al. . The "Reading the Mind in the Eyes" Test: Complete Absence of Typical Sex Difference in ~400 Men and Women with Autism. PLoS One 2015;10: e0136521. [DOI] [PMC free article] [PubMed] [Google Scholar] 48. Andreeva E., Brenner M.H., Theorell T.et al. . Risk of psychological ill health and methods of organisational downsizing: a cross-sectional survey in four European countries. BMC public health 2017;17: 758. [DOI] [PMC free article] [PubMed] [Google Scholar] 49. Zuelke A.E., Luck T., Schroeter M.L.et al. . The association between unemployment and depression-Results from the population-based LIFE-adult-study. J affective Disord 2018;235: 399–406. [DOI] [PubMed] [Google Scholar] 50. Andreeva E., Magnusson Hanson L.L., Westerlund H.et al. . Depressive symptoms as a cause and effect of job loss in men and women: evidence in the context of organisational downsizing from the Swedish Longitudinal Occupational Survey of Health. BMC public health 2015;15: 1045. [DOI] [PMC free article] [PubMed] [Google Scholar] 51. Piontek A., Szeja J., Blachut M.et al. . Sexual problems in the patients with psychiatric disorders. Wiad Lek 2019;72: 1984–1988. [PubMed] [Google Scholar] 52. Pyke R.E. Sexual Performance Anxiety. Sex Med Rev 2020;8: 183–190. [DOI] [PubMed] [Google Scholar] 53. Kaplan H.S. Anxiety and sexual dysfunction. J Clin Psychiatry 1988;49: Suppl: 21–25. [PubMed] [Google Scholar] 54. Love T.M. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav 2014;119: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar] 55. Ciocca G., Limoncin E., Carosa E.et al. . Is Testosterone a Food for the Brain? Sex Med Rev 2016;4: 15–25. [DOI] [PubMed] [Google Scholar] 56. Carosa E., Benvenga S., Trimarchi F.et al. . Sexual inactivity results in reversible reduction of LH bioavailability. Int J Impotence Res 2002;14: 93–99. [DOI] [PubMed] [Google Scholar] 57. Veening J.G., de Jong T.R., Waldinger M.D.et al. . The role of oxytocin in male and female reproductive behavior. Eur J Pharmacol 2015;753: 209–228. [DOI] [PubMed] [Google Scholar] 58. Corona G., Jannini E.A., Vignozzi L.et al. . The hormonal control of ejaculation. Nat Rev Urol 2012;9: 508–519. [DOI] [PubMed] [Google Scholar] 59. Corona G., Jannini E.A., Lotti F.et al. . Premature and delayed ejaculation: two ends of a single continuum influenced by hormonal milieu. Int J Androl 2011;34: 41–48. [DOI] [PubMed] [Google Scholar] 60. Jannini E.A., Screponi E., Carosa E.et al. . Lack of sexual activity from erectile dysfunction is associated with a reversible reduction in serum testosterone. Int J Androl 1999;22: 385–392. [DOI] [PubMed] [Google Scholar] 61. Spitzer M., Basaria S., Travison T.G.et al. . Effect of Testosterone Replacement on Response to Sildenafil Citrate in Men With Erectile Dysfunction. Ann Intern Med 2012;157: 681–691. [DOI] [PubMed] [Google Scholar] 62. Fabbri A., Jannini E.A., Ulisse S.et al. . Low Serum Bioactive Luteinizing Hormone In Nonorganic Male Impotence: Possible Relationship with Altered Gonadotropin-Releasing Hormone Pulsatility. J Clin Endocrinol Metab 1988;67: 867–875. [DOI] [PubMed] [Google Scholar] 63. Jannini E.A., Fisher W.A., Bitzer J.et al. . Controversies in Sexual Medicine: Is Sex Just Fun? How Sexual Activity Improves Health. The J Sex Med 2009;6: 2640–2648. [DOI] [PubMed] [Google Scholar] 64. Corona G., Mannucci E., Lotti F.et al. . Impairment of Couple Relationship in Male Patients with Sexual Dysfunction is Associated with Overt Hypogonadism. The J Sex Med 2009;6: 2591–2600. [DOI] [PubMed] [Google Scholar] 65. Laumann E.O., Nicolosi A., Glasser D.B.et al. . Sexual problems among women and men aged 40-80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res 2005;17: 39–57. [DOI] [PubMed] [Google Scholar] 66. Corona G., Lee D.M., Forti G.et al. . Age-related changes in general and sexual health in middle-aged and older men: results from the European Male Ageing Study (EMAS). J Sex Med 2010;7: 1362–1380. [DOI] [PubMed] [Google Scholar] 67. Hayes R., Dennerstein L.. The impact of aging on sexual function and sexual dysfunction in women: a review of population-based studies. J Sex Med 2005;2: 317–330. [DOI] [PubMed] [Google Scholar] 68. Laumann E.O., Paik A., Rosen R.C.. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281: 537–544. [DOI] [PubMed] [Google Scholar] 69. Rosen R.C., Fisher W.A., Eardley I.et al. . The multinational Men’s Attitudes to Life Events and Sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin 2004;20: 607–617. [DOI] [PubMed] [Google Scholar] 70. Hayes R.D., Dennerstein L., Bennett C.M.et al. . What is the "true" prevalence of female sexual dysfunctions and does the way we assess these conditions have an impact? J Sex Med 2008;5: 777–787. [DOI] [PubMed] [Google Scholar] 71. Qiu J., Shen B., Zhao M.et al. . A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatr 2020;33: e100213. [DOI] [PMC free article] [PubMed] [Google Scholar] 72. Wang C., Pan R., Wan X.et al. . Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health 2020;17: [DOI] [PMC free article] [PubMed] [Google Scholar] 73. Wittchen H.U., Nelson C.B., Lachner G.. Prevalence of mental disorders and psychosocial impairments in adolescents and young adults. Psychol Med 1998;28: 109–126. [DOI] [PubMed] [Google Scholar] 74. Stordal E., Mykletun A., Dahl A.A.. The association between age and depression in the general population: a multivariate examination. Acta Psychiatr Scand 2003;107: 132–141. [DOI] [PubMed] [Google Scholar] 75. Altschul D., Iveson M., Deary I.J.. Generational differences in loneliness and its psychological and sociodemographic predictors: an exploratory and confirmatory machine learning study. Psychol Med 2020. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar] 76. Marziali M.E., Armstrong H.L., Closson K.et al. . Loneliness and self-rated physical health among gay, bisexual and other men who have sex with men in Vancouver, Canada. J Epidemiol Community Health 2020;74: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar] 77. Menec V.H., Newall N.E., Mackenzie C.S.et al. . Examining social isolation and loneliness in combination in relation to social support and psychological distress using Canadian Longitudinal Study of Aging (CLSA) data. PLoS One 2020;15: e0230673. [DOI] [PMC free article] [PubMed] [Google Scholar] 78. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat 2011;7: 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar] 79. Esch T., Stefano G.B.. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett 2004;25: 235–251. [PubMed] [Google Scholar] 80. Esch T., Stefano G.B.. The Neurobiology of Love. Neuro Endocrinol Lett 2005;26: 175–192. [PubMed] [Google Scholar] 81. Kramer J., Rubin A., Coster W.et al. . Strategies to address participant misrepresentation for eligibility in Web-based research. Int J Methods Psychiatr Res 2014;23: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar] 82. Bradley D.T., Mansouri M.A., Kee F.et al. . A systems approach to preventing and responding to COVID-19. EClinicalMedicine 2020;21: 100325. [DOI] [PMC free article] [PubMed] [Google Scholar] 83. Lunn P.D., Belton C.A., Lavin C.et al. . Using Behavioral Science to help fight the Coronavirus. J Behav Public Adm 2020;3: 1–15. [Google Scholar]Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1
Supplementary table 2